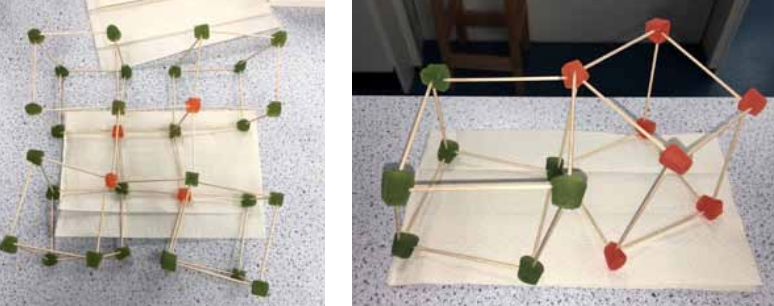
Last week in ChemSoc, we explored the idea of using models to explain complex ideas, specifically atomic structure. For as circles around a nucleus, and at A level, we delve deeper and begin to look at the idea of orbitals. But last week we tested an atomic model created in the past by a chemist called Gilbert Lewis, which represents electrons at the eight corners of a cube. We explored this model using toothpicks and gummy sweets, and found that although it worked for some molecules, like chlorine and tetrachloromethane, which are pictured above, we found that it didn’t work for all of them. This also sparked a discussion on how science and our understanding of it is continually changing, when it is time to refine or completely replace the models that we use, and how the models we currently use to represent atomic structure could change in the future.
Julieta, Year 12
